   |
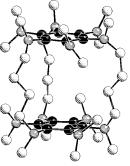
Hypercloso-type boron subhalides
Most boron clusters follow Wade's rules of electron count and have closo structures for 2n + 2
cage bonding electrons. Boron subhalides of type BnXn
(X = Hal, n = 6,8-12) are an exception. They possess only 2n electrons in the
cluster framework, but still show closo structures (hypercloso-clusters). Coworkers in this project:Carsten Tittel, Tina Wizemann and Marc WürdeThis project is based on a cooperation with Wolfgang Einholz. We thank W. Preetz, Kiel, for samples of B6X62- salts. Oral presentations:Bernd Speiser at thePublications from this project:B. Speiser, T. Wizemann und M. Würde, Inorg. Chem., 42, 4018 - 4028 (2003); Two-Electron-Transfer Redox Systems. Part 7. Two-Step Electrochemical Oxidation of the Boron Subhalide Cluster Dianions B6X62- (X = Cl, Br, I).W. Einholz, K. Vaas, C. Wieloch, B. Speiser, T. Wizemann, M. Ströbele und H.-J. Meyer, Z. anorg. allgem. Chem., 628, 258 - 268 (2002); Zweielektronentransfer-Redoxsysteme, Teil 5. Chemische und cyclovoltammetrische Untersuchung der Redoxreaktionen der Decahalogendecaborate closo-[B10X10]2- und hypercloso-[B10X10]•- (X = Cl, Br). Kristallstrukturanalyse von Cs2[B10Br10] . 2 H2O. B. Speiser, C. Tittel, W. Einholz und R. Schäfer, J. Chem. Soc., Dalton Trans., 1999, 1741 - 1751; Two-Electron-Transfer Redox Systems. Part 2. Redox Reactions of the Boron Subhalide Clusters BnCln0/•-/2- (n = 8,9) Investigated by Electrochemical and Spectroscopic Methods.
|
| © AG Speiser 2011 | |